The Philippine Food and Drug Administration (FDA) recently announced the approval of oral medicine Olaparib for the treatment of various types of cancers such as patients with Advanced Ovarian, Breast, Prostate, and Pancreatic Cancer. AstraZeneca Philippines brings this drug to the country, celebrating this huge milestone for Filipinos with a stronger push to promote early doctor consultation to assess cancer risk and get treated.
Cancer remains to be one of the leading causes of death in the Philippines, with the Department of Health (DOH) stating that 189 of every 100,000 Filipinos are afflicted by cancer, while four Filipinos die of the terminal illness every hour, amounting to 96 cancer patients every day.2 The approval of Olaparib comes with the hopeful promise to rapidly aid the mitigation of these numbers across the country.
The FDA approval in the Philippines was based on the results of the SOLO-1, Study 19, SOLO-2, and PAOLA-1 trials for ovarian cancer3, the OlympiAD trial for breast cancer4, the POLO trial for pancreatic cancer5, and the PROfound trial for prostate cancer6. These studies confirm the drug’s significant contribution to cancer treatment, based on its many therapeutic indications for several cancer types.
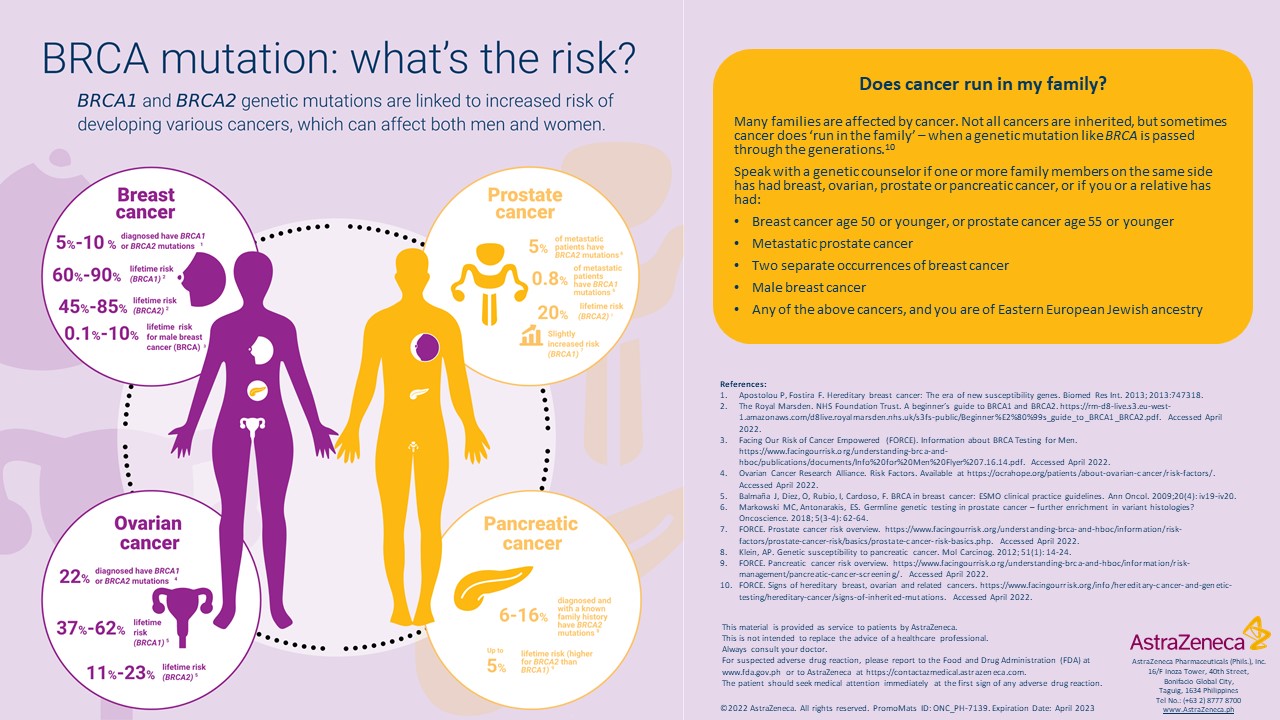
BRCA1 and BRCA2 are two genes that are important to fighting cancer. When mutations in these genes are present, cancer cells grow and divide too rapidly or in an uncontrolled way. Poly (ADP ribose) polymerase (PARP) are enzymes that act as back up to BRCA1 and BRCA2 in the efficient repair of DNA. PARP helps cells, including cancer cells, survive and grow7. Olaparib inhibits the growth of cancer because it is a potent inhibitor of PARP.8
Meaningful Treatment for Cancer Patients
Dubbed as a “revolutionary drug”, Olaparib’s list of indications include maintenance treatment to keep the results of successful chemotherapy for ovarian and pancreatic cancer, and active treatment to help shrink or slow down the growth of tumors in breast and prostate cancer patients.9 As there are currently limited treatment options for these cancer types, the approval of Olaparib comes at no better timing as it significantly improves patient care today.
“It is exciting to finally have Olaparib, an oral cancer medicine, to be locally available. It will benefit patients with various types of advanced disease: breast, ovarian, pancreatic, and prostate cancers,” adds Dr. Gerardo Cornelio, Medical Oncologist, Director of Cancer Institute, St. Luke’s Medical Center Global City and Past Chair, San Juan De Dios Oncology Unit.
Dr. Maria Lilibeth Sia Su, Professor and Consultant of Gynecologic Oncology of UPCM-PGH shares,
“The long wait for the availability of Olaparib in our country is finally over. The clinical trials on Olaparib for advanced epithelial ovarian cancer is very exciting as this drug offers our patients a chance for better progression-free survival (PFS) and improved overall survival (OS) with fewer complications. My patient, now on her sixth month of Olaparib has not reported any adverse event.”
The excited sentiment is shared by patients and doctors alike for the drug’s long-awaited arrival in the Philippines. A Filipino patient with ovarian cancer who wishes to remain anonymous also commented, “I felt like I had cancer in the wrong country because we didn’t have this medicine. Having it available here gives me hope.”
Better Care Today
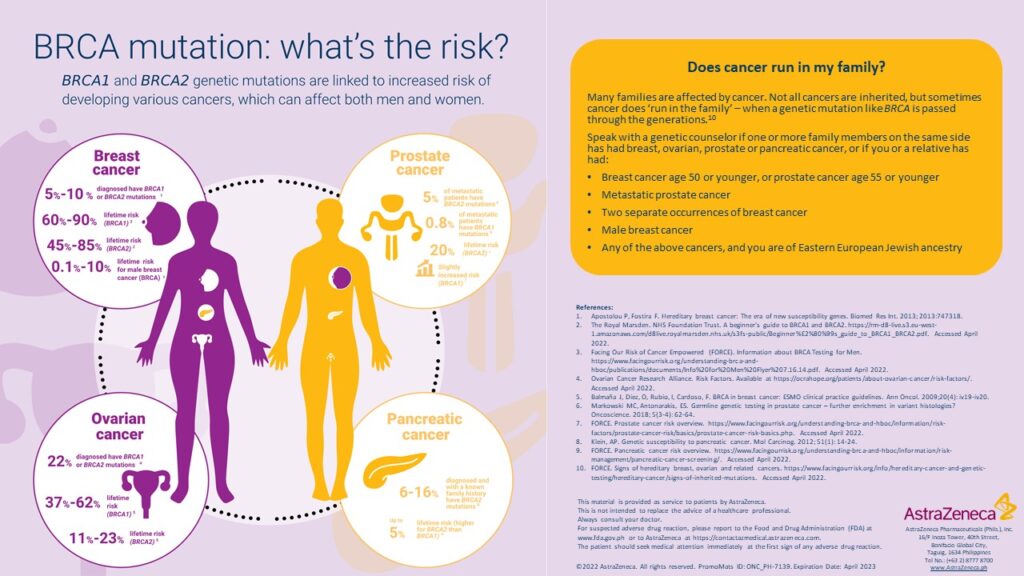
Just like Angelina Jolie, Filipino cancer patients as well as healthy people who have strong cancer family history are now prompted to get tested and know their risk of having BRCA mutations, to assess their compatibility with the Olaparib oral drug and understand their risk for developing cancers. Consultations with their doctors are highly encouraged to discuss ongoing programs that can further help their treatment.
In light of this, AstraZeneca Philippines advocates its AZ Cares Patient Access Program with renewed fervor, encouraging Filipinos to regularly consult their doctors to maintain physical health, especially when it comes to detecting and treating cancer early.
Lotis Ramin, country president of AstraZeneca Philippines, remarks “A first-in-class PARP inhibitor, this important approval of Olaparib gives high-risk patients with certain types of ovarian, breast, as well as pancreatic and prostate cancers a new targeted therapy option that confers unprecedented survival benefits vs. standard of care1. As an industry leader, AstraZeneca is proud to forge innovative solutions to improve diagnosis of patients and make Olaparib treatment accessible to Filipino cancer patients.”
Olaparib is a prescription medicine. Patients should not self-medicate and should consult their physicians regarding their condition and available treatment options and information on contraindications, precautions, warnings and/or side effects.
To learn more about AstraZeneca’s commitment to healthcare, go to https://www.astrazeneca.com/.